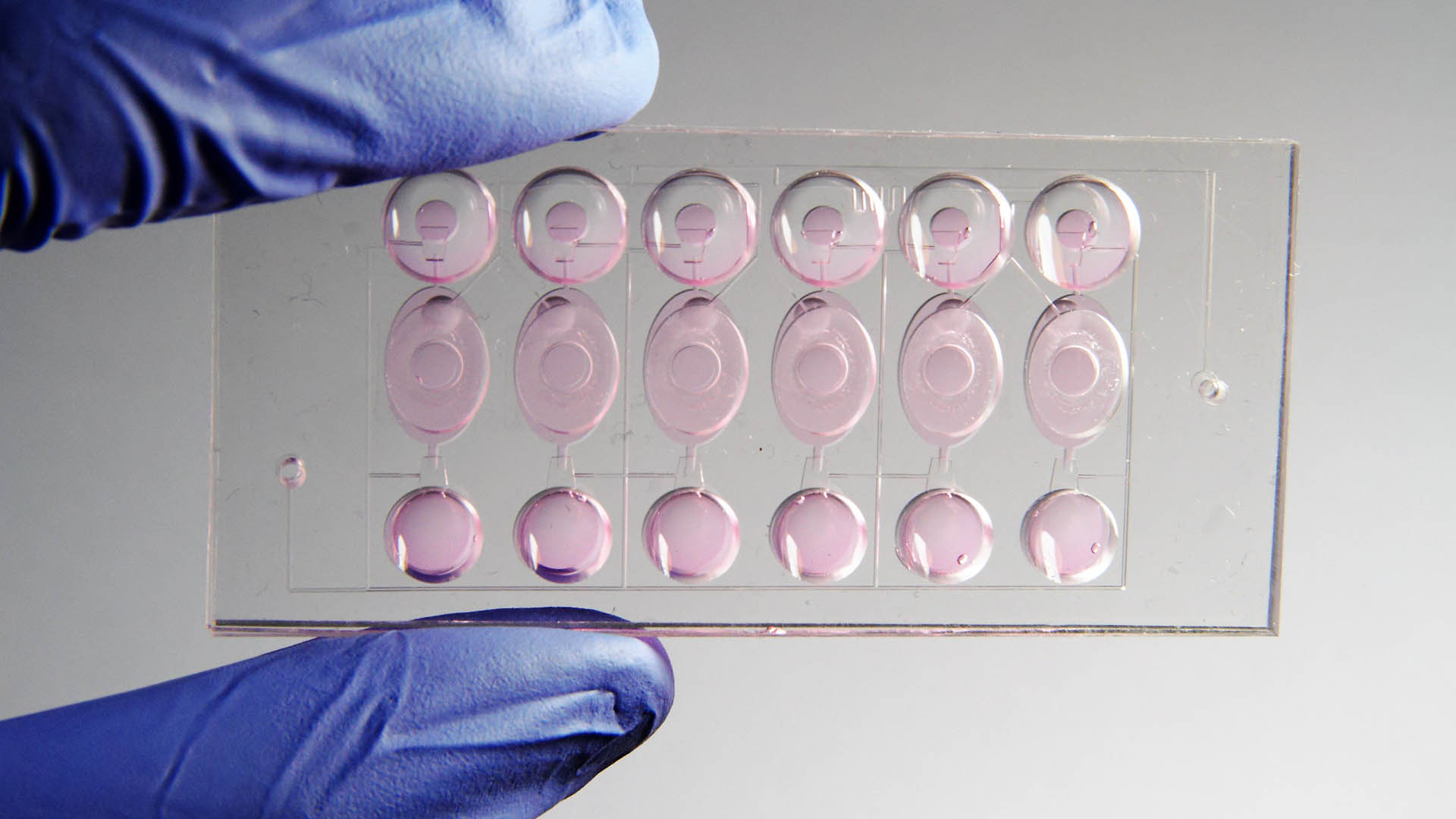
ABOUT ANIMAL TESTING AND THE 3R PRINCIPLES
In Switzerland, animal experiments are limited to an absolute minimum in accordance with the Animal Protection Act: They may only be carried out if no suitable alternative method is known with which the desired goal can also be achieved. Researchers must justify each animal experiment and prove that the benefit society derives from the planned animal experiment justifies the distress to the animals during the experiment. This so-called balancing of interests is a central element in the authorization procedure. The 3R principle comprises three tools that enable the maximum protection of animals used in experiments without diminishing the conclusiveness of scientific research: -Replace: Replacing an animal test as far as possible if there are suitable alternative methods. -Reduce: The reduction of animal experiments and the number of test animals as far as possible. The decisive factor here is to use as many animals as are necessary for a statistically reliable statement. A sufficient number of animals must be used, otherwise the results would be too inconclusive. -Refine: The methods and handling of animals during experiments and in captivity should ensure that their distress is kept to a minimum, enhancing animal welfare.